May 13. 2019 13:26 GTM
“Bifidobacterium breve Bif195 protects against small intestinal damage caused by Acetylsalicylic Acid in healthy volunteers’ is a clinical trial that demonstrates the probiotic strain, Bif195, strengthens the defense against gut damage caused by regular use of aspirin.
“Gastroenterology is the leading journal within the field of gut and gastro health and we are delighted that this study has been accepted by such a highly reputed journal. The publication of the clinical study is a crucial step in the process towards commercialization of this strain as a new dietary supplement. Our end goal is to bring a solution that supports the defense against damage to the gut,” says Thomas Riis Jensen, head of commercial development in Human Health.
The publication of the clinical trial is now available on Gastroenterology’s website.
Intestinal damage caused by regular use of aspirin
The daily intake of low-dose aspirin can reduce the risk of heart disease and is taken by approximately 40 million people in the US alone1. However, the regular use of aspirin can have several side effects. Almost half of these 40 million people have tissue damage in the gut, including ulcers2. There is currently no product on the market that shows a documented scientific effect of helping to protect against this.
In a proprietary consumer survey on aspirin users, 50% of respondents voiced concern about gut-related side effects, including gastrointestinal ulcers, upset stomach and abdominal pain3.
New probiotic strain can protect the intestine
In this randomized placebo-controlled clinical trial with healthy volunteers, the results show that daily oral intake of Chr. Hansen’s specially selected strain, Bif195, can help protect against intestinal damage induced by aspirin.
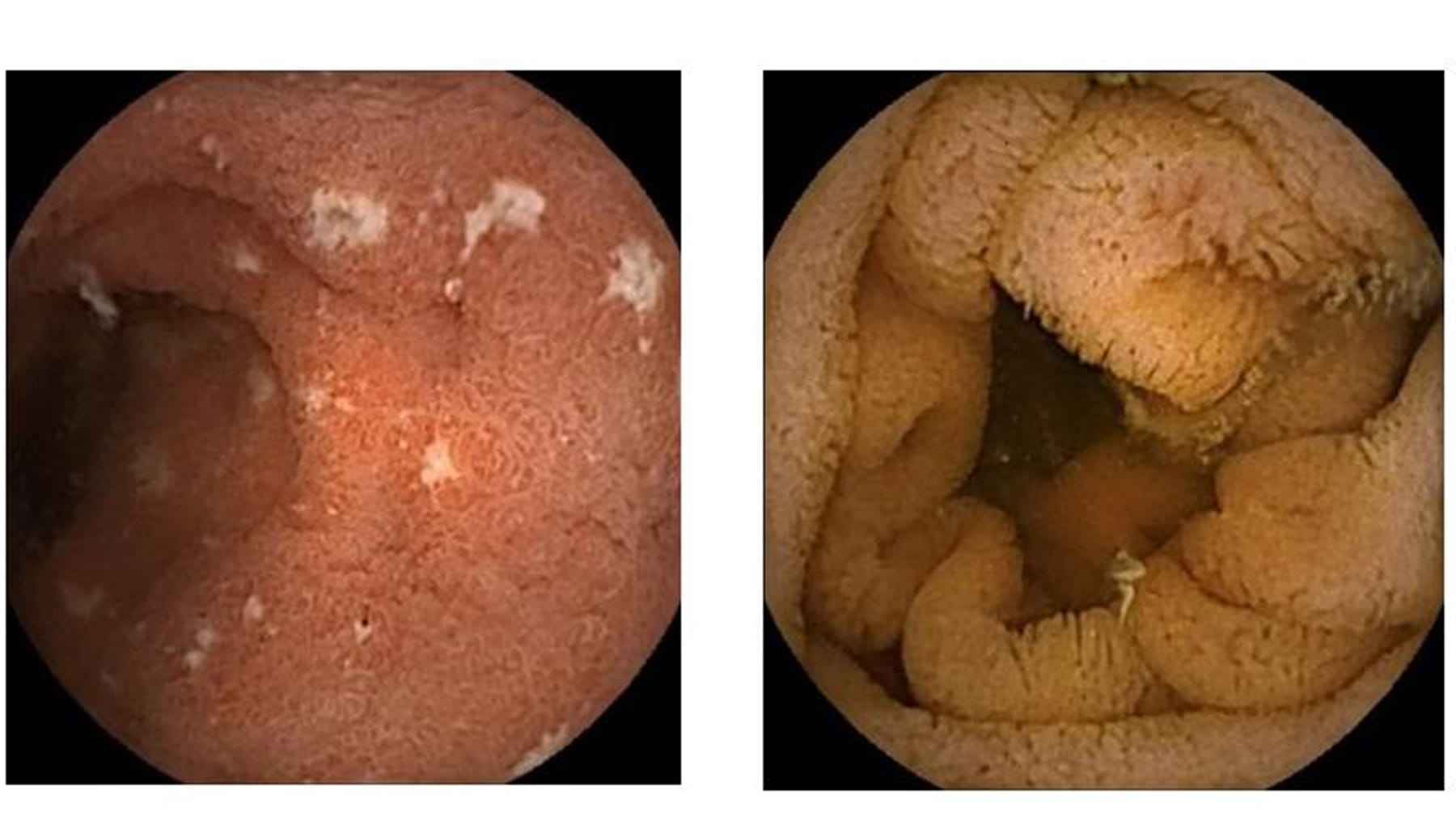
Pictures from the clinical trial of the small intestine. Aspirin and placebo on the left showing edema and lack of intestinal villi; Aspirin and Bif195 on the right showing a normal small intestine with villi. Mortensen et al. 2019, Gastroenterology, in press.
“We used the advanced technology ‘video capsule endoscopy’ to obtain an objectively verifiable measure of the clinical effects. As well as clearly showing the reduction of risk of ulcers on the small intestine, the daily oral intake of Bif195 is safe and without side effects,” explains Anders Damholt, lead scientist on the project in Human Health Innovation.
Your health – our science
With the clinical trial showing significant effects of Bif195, Chr. Hansen is now in the early stage of a new product development.
“We continue to invest in both science and manufacturing related to this carefully selected proprietary strain, as we see a strong need for such a solution in the market. We have additional clinical trials in the pipeline to further confirm the data and we are working on production processes. Meanwhile, we are in dialogues with potential partners for the commercialization of this dietary supplement. Our goal is to make this new strain available to the many millions who need to take aspirin regularly to support their long-term health,” concludes Riis Jensen.
References
1 Chr. Hansen consumer survey, December 2018.
2 J Multidiscip Healthc. 2014, 7:137–146.
3 Chr. Hansen consumer survey, December 2018.
Chr. Hansen is a global, differentiated bioscience company that develops natural ingredient solutions for the food, nutritional, pharmaceutical and agricultural industries. At Chr. Hansen we are uniquely positioned to drive positive change through microbial solutions. We have worked for almost 150 years to enable sustainable agriculture, better food and healthier living for more people around the world. Our microbial and fermentation technology platforms, including our broad and relevant collection of around 50,000 microbial strains, have game-changing potential. Matching customer needs and global trends we continue to unlock the power of good bacteria to respond to global challenges such as food waste, global health and the overuse of antibiotics and pesticides. As one of the world’s most sustainable companies, we touch the lives of more than 1 billion people every day. Driven by our legacy of innovation and curiosity to pioneer science, our purpose – To grow a better world. Naturally. – is at the heart of everything we do.