No hidden surprises for your products, brands or business
For any functional food or dietary supplement provider not prepared to make a single compromise in selecting a probiotic with proven benefits in a range of health areas, there can only be LGG®.
The world’s most documented probiotic strain
Since 1985, Lacticaseibacillus rhamnosus, LGG® has been the subject of more than 2,000 scientific publications, including 300 publications of human studies. LGG® has been used worldwide since 1990 as an ingredient in food and dietary supplements with no safety issues.
This wealth of clinical documentation demonstrates the strain’s benefits and safety across life stages and health areas, from new-borns1, children2, 3, 4 pregnant women5, 6, adults7 and the elderly8.
Proven genomic stability
Using state-of-the-art sequencing technology, we have generated a closed genome reference sequence of the LGG® probiotic strain. These results show that the LGG® probiotic strain has remained stable and genomically identical to the strain first isolated in 1985.16,17 The genome results and sequences have been validated and endorsed by an independent third party.
Superior survivability
- Lab-based research has shown that the LGG® probiotic strain has high acid and bile tolerance, which may be important for a probiotic strain to survive passage through the gastrointestinal tract and for its potential beneficial effects.18
- The LGG® strain supports intestinal barrier function by helping intestinal integrity and supporting the immune system.18
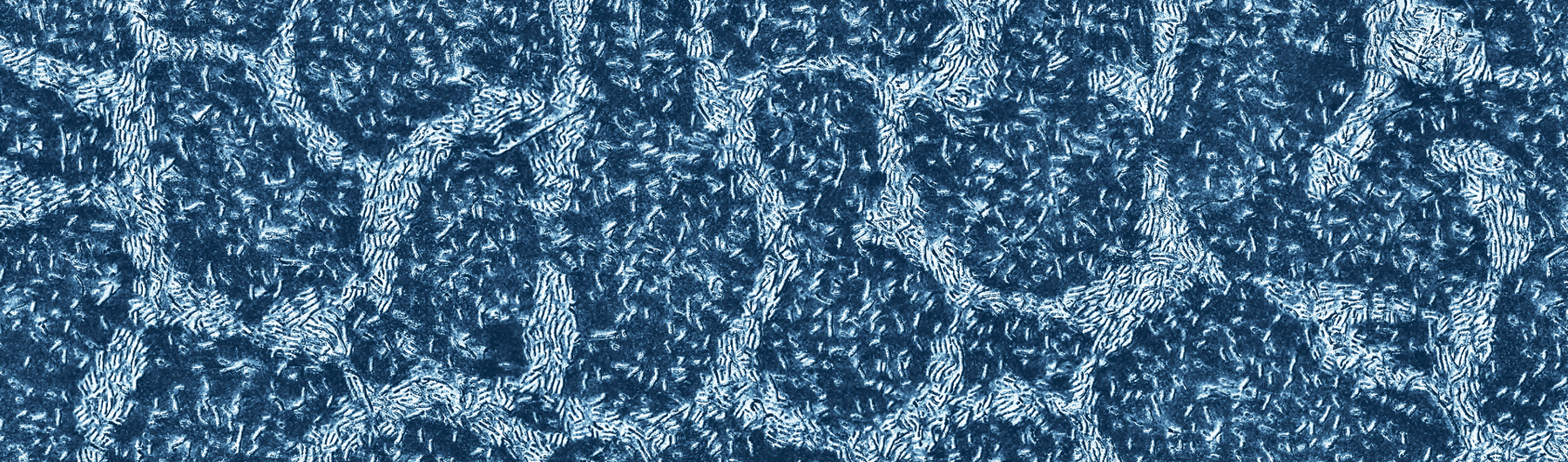
- It has also been seen that LGG® exhibits strong capacity for mucus adhesion.19, 20 It is believed that pili, hair-like appendages, are crucial for adhesion to the intestinal mucosa.21
- LGG® boasts uniform pili length and density when viewed under a microscope.
A production process protected by 100 patents and applications
Our strictly controlled production process ensures the consistency, stability and integrity of the LGG® strain remain unmatched.
- In dietary supplements, LGG® shows superior stability for up to 36 months.*
- We have maintained the same strain via controlled storage and renewal of strain cell banks, and we apply our unique know-how on fermentation and freeze drying to produce the same LGG® product with high quality every time.
- Our state-of-the-art production processes ensure pili integrity, which allow for increased adhesion in the gastrointestinal tract.
* LGG® is stable in a wide selection of fermented dairy and plant based dairy alternative products
The LGG® probiotic strain is safe for human consumption and has been granted QPS (Qualified Presumption of Safety) status in Europe22 and been the subject of a GRAS (Generally Recognized As Safe) notice to the US Food and Drug Administration,23 with no safety issues.
LGG® is a registered trademark of Chr. Hansen A/S.
References
1 Arvola T, et al. Pediatrics. 1999;104(5):e64.
2 Vanderhoof JA, et al. The Journal of Pediatrics. 1999;135(5):564-8.
3 Hojsak I, et al. Pediatrics. 2010;125(5):e1171-7.
4 Isolauri E, et al. Pediatrics. 1991;88(1):90-7.
5 Gueimonde M, et al. Journal of Pediatric Gastroenterology and Nutrition. 2006;42(2):166-70.
6 Lahtinen SJ, et al. J Allergy Clin Immunol. 2009;123(2):499-5019.
7 Hilton et al.
8 Hatakka K, et al. J Dent Res. 2007;86(2):125-30.
9 Hojsak I, et al. Clin Nutr. 2010;29(3):312-6.
10 Davidson LE, et al. Eur J Clin Nutr. 2011;65(4):501-7.
11 Sindhu KNC, et al. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(8):1107-15.
12 Aggarwal S, et al. Indian J Med Res. 2014;139(3):379-85.
13 Nase L, et al. Caries Res. 2001;35(6):412-20.
14 Poerksen et al., J Dent. 2023;135:104599.
15 Glavina D, et al. Coll Antropol. 2012;36(1):129-32.
16 Vos WM. 2009. Proc Natl Acad Sci United States Am 106:17193 –17198.
17 Stage M, et al., Appl Environ Microbiol. 2020; 86(6)e02780-19.
18 Ref: data from Chr. Hansen Human Health Research.
19 Tuomola et al. 1999.
20 Lebeer et al. 2012.
21 Rasinkangas et al. 2014.
22 EFSA Panel on Biological Hazards (BIOHAZ). EFSA Journal. 2015;13:4331.
23 Food and Drug Administration. GRAS Notice No GRN 000049. 2002.
This communication is only intended for business-to-business and health care professionals. This communication is not intended for consumers of final consumer goods. Nothing on this page is meant to be perceived as an approved claim.